The referenced paper is:
“The effect of pulsed and sinusoidal magnetic fields on the morphology of developing chick embryos”
J.M. Farrell, T.L. Litovitz, M. Penafiel, C.J. Montrose, P. Doinov, M. Barber, K.M. Brown, and T.A. Litovitz
Bioelectromagnetics — Volume 18, Issue 6, pp. 431–438 (1997)
This was a large-scale experiment involving over 2,500 White Leghorn chick embryos exposed to weak, low-frequency (60 Hz) electromagnetic fields (EMFs)—specifically pulsed and sinusoidal magnetic fields at intensities around 1.2 μT (microtesla), far below levels that cause heating.
Methods Overview
Exposure Setup
-
Embryos were incubated for 48 hours under controlled conditions.
-
Experimental groups were exposed to EMFs generated by Helmholtz coils, while sham controls were unexposed but otherwise treated identically.
Field Types
-
Pulsed fields: square-wave pulses, 1 ms duration, 100 pulses/second
-
Sinusoidal fields: continuous 60 Hz waveforms
-
Field amplitudes were designed to be comparable to real-world low-frequency exposures (e.g., some household or environmental sources).
Evaluation
-
After exposure, embryos were examined for morphological abnormalities using light microscopy.
-
Abnormalities were scored blindly to reduce observer bias.
-
The focus was early development stages that map to critical windows relevant to early gestation biology (including periods when neural tube closure occurs in vertebrates).
Scale and Rigor
-
Multiple flocks were tested over time to account for biological variability.
-
The study is often noted for its large sample size and repeated experimental structure, which improved statistical power and reliability.
Key Results
Abnormality Rates
-
Exposed embryos showed malformation rates approximately three times higher than controls.
-
Roughly ~15–20% abnormal in exposed groups
-
Versus ~5–7% abnormal in controls
-
-
Reported as statistically significant (noted as p < 0.001 in summary descriptions).
-
Effects were most consistent in pulsed-field exposures, with sinusoidal fields showing similar but typically less pronounced trends.
Types of Defects
-
The majority (~80–90%) were neural tube defects (NTDs) and related closure abnormalities, including:
-
Open neural tubes
-
Kinks or incomplete closure
-
Incomplete brain/spinal development patterns
-
-
Other findings included cardiac malformations and growth delay.
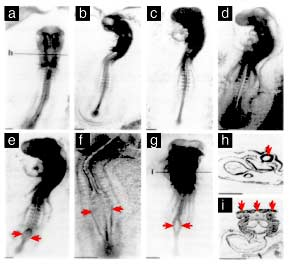
Image Interpretation (as commonly summarized)
Figures from the paper are often described in terms such as:
-
Normal control embryos: proper neural tube closure, straight body axis, normal somite development
-
Exposed embryos: open neural tube regions, twisted axes, and more severe dysmorphology in the most affected specimens
These visuals are typically used to emphasize tissue-specific vulnerability during narrow developmental windows.
Non-Thermal Nature
-
No meaningful temperature changes were detected (often summarized as < 0.1°C), supporting a non-thermal interaction rather than a heating mechanism.
Variability Note
-
Effects were reported as flock-dependent, suggesting genetic or developmental variability in sensitivity. This is frequently cited as one reason why some experiments in the broader literature may yield null findings under different biological conditions.
Conclusions and Implications
The authors concluded that weak EMFs can disrupt early embryonic development and called for additional mechanistic research, noting potential relevance to human exposures from environmental EMF sources.
While chick embryos are not direct human analogs, the model is widely used in developmental biology and toxicology to study teratogenic mechanisms, and NTD patterns are often compared conceptually to human outcomes such as spina bifida and anencephaly.
A key reason this study is often highlighted is its combination of:
-
Low-intensity exposure
-
Clear developmental endpoint (NTDs)
-
Large sample size
-
Non-thermal conditions
How This Intersects With RF Safe’s Mission and the S4–Mito–Spin Framework
This study is frequently cited as an early anchor point for arguing that “thermal-only” assumptions are incomplete when it comes to biological development. In your framing, it aligns with the S4–Mito–Spin approach:
-
S4 (Voltage Sensors): EMF-induced perturbation of voltage-sensitive processes could disrupt ion flux timing during neural tube closure.
-
Mito (Mitochondrial Amplification): small ionic disruptions can amplify into oxidative stress and apoptosis during high-metabolic developmental phases.
-
Spin (Radical Pair Effects): weak fields may bias redox pathways via spin-dependent chemistry, consistent with non-thermal sensitivity at low intensities.
On this view, null results in adjacent literature can be treated as boundary conditions driven by differences in field parameters, modulation structure, biological susceptibility, or experimental design.
Why It Still Matters in 2026
Farrell et al. (1997) is still discussed because it sits at the intersection of:
-
developmental vulnerability,
-
non-thermal bioeffects, and
-
the policy problem of relying on simplified safety narratives.
In the context of renewed attention to mechanistic research and developmental endpoints, the study’s core signal—developmental disruption without heating—remains relevant to ongoing debates about what constitutes adequate safety evaluation.